ALL THINGS KOI
AND H2O
BLOOD RELATIONS
By Duncan Griffiths
The
physiology of koi carp is a fantastic topic; none more so than the subject
of gas transfer. Once one gets involved in this kind of study, it soon becomes
apparent that the fish are a miracle of design that have adapted to an environment
(WATER) that is a real challenge for a living organism.
Oxygen is the basis for most life forms to survive.
We as human beings breathe in atmosphere that contains about 20% oxygen.
Oxygen is relatively insoluble in water and as such fishes live in an environment
that can typically carry a maximum O2 content about 30 times less
than the atmosphere, this places the fish at a distinct disadvantage. But where as humans living in a relatively
oxygen rich environment, we can only extract 20% of that available oxygen
where as fish can extract 80%.
In humans our bodily waste is excreted in the
form of uric acid and faeces and CO2,
Although
fish excrete faeces they cannot afford the luxury of excreting uric acid as
this would in the end acidify their environment and continually reduce the
pH, and result in their down fall, so fish produce ammonia instead, which
natural bacteria can detoxify for them and the action to deal with this, at
least this requires no effort / energy on the part of the fish.
Despite these obvious disadvantages,
fish evolution has adapted and over come these obstacles to such an extent
they can live in quite a range of aquatic conditions, from very high oxygen
content of water typical found in streams and rivers, to ponds, lakes and
swamps with poor oxygen saturation.
Antarctic ice fish have adapted that well to
low temperatures to such an extent that they no long have haemoglobin, the
basic oxygen carrying molecule that is found in the blood of all animals and
certainly essential for support of life as we know it.
If we first consider oxygen transport around
the body, there is in no way that enough oxygen can be held in simple solution
in cool to warm water to support a fish’s life. So fish have evolved the physiology to cope with the low availability
of oxygen in the surrounding water.
The
gas exchange apparatus is the gill. This
organ has a very thin membrane only one cell thick to allow the blood to be
in as close contact to the oxygenated water as possible.
Oxygen crosses this membrane into the blood plasma
by a process called diffusion. Diffusion
is when a solute, in this case oxygen, migrates from an area of higher concentration
to an area of lower concentration in an attempt to equalise the concentration/pressure.
To visualize this, imagine a pond full of clear still water to which
is very carefully added one cup of malachite green solution. Very soon it will start to spread out and continue until the whole
of the pond is equally saturated with malachite green. This is the malachite green molecules diffusing
to the lower concentrations, i.e., to the clear-water portions of the pond.
Essentially this how oxygen moves across the gill membrane, i.e., the
pond is the higher concentration and the plasma in the gill is the lower concentration.
However as previously mentioned, the pond is
saturated in oxygen to a maximum of around 11 ppm, dependant on temperatures.
This concentration is not enough to sustain a fish so the fish has
to do other things to increase this concentration and indeed as a couple of
tricks up its sleeve to achieve this.
Blood as we know it is just a transport media,
pure and simple, it is responsible for the transport of Oxygen and Carbon
Dioxide to and from the lungs or gills in the case of fish, to respiring tissue,
this is caried in the haemoglobin and hydrogen-carbonate ions in the plasma.
It further transports organic digestive products, I.E. Glucose Amino Acids
and vitamins from the intestine to respiring tissue and liver, this time in
the plasma. Mineral salts, I.E. Calcium, Iodine, Iron from the intestine to
the bones, teeth, thyroid gland. Hormones, from the Pituitary gland to the
liver, it even transports a certain amount of metabolic heat, less so in fish
but more so in mammals, the list goes on but
you can see when it comes down to basic's, it’s simply a transport
media.
Blood is made up of white blood cells (Leucocytes) these contain the
bodies defence and immune system, they are ideally situated here to fight
disease and antigens as they can reach any part of the body. ,(Erythrocytes),
Red blood cells. (Platelets),
these contain amongst other things clotting agents, a koi's blood is very
high in clotting factor and clots extremely fast. Finally the Plasma, the
clear transport fluid/media containing mineral salts hydrogen and carbonate ions, iodine, etc.
However, the red blood cells Erythrocytes and Plasma, are what
we are concerned with. The red blood
cells, Erythrocytes, are the essential oxygen carrying agents.
Blood arrives at the gill depleted of oxygen but carrying CO2,
carbon dioxide and carbonic acid - the by-product
of metabolism, ready to take on board a fresh supply of O2. As this newly arrived blood has little
or no oxygen, the concentration of O2 is very low or nil and oxygen
will diffuse to across the gill lamellae from the higher concentration in
the pond to this low concentration in the blood plasma. However as previously
mentioned a fully loaded state of equilibrium (oxygen in solution) can only
achieve a maximum status of 10/11 ppm and that would not be enough for the
fishes life support needs. If this
saturation is the best the fish can achieve, the fish may as well have pond
water flowing through its veins.
However,
floating in the blood plasma are red blood cells and these contain haemoglobin
molecules. Each haemoglobin molecule
is capably of chemically bonding with four oxygen molecules thus taking the
oxygen out of the pressure equation of the plasma.
This allows the plasma to diffuse yet more valuable oxygen. And, almost as fast as the oxygen diffusion
takes place, the haemoglobin binds it.
To explain further, a red blood cell (Erythrocyte) shape is round and is termed biconcave, but far from being ball shaped, it is concave on each side to maximize surface area,
contained in this is the respiratory pigments, which are proteins. Contained
within the red blood cells are haemoglobin molecules each of which is made
up of four sub groups, or Haem group. Each of these sub groups contain polypeptides
made up of around 140 amino acids and ferrous iron, the iron amongst other
things, gives blood its red colour. If the haemoglobin molecules were just
in suspension in the plasma they would be excreted, during ultra-filtration
by the kidney, hence the reason why they are locked away inside the red blood
cells, erythrocytes. It is the iron content that is responsible for
attracting oxygen and binding with the oxygen, until time comes to release
it into the tissue. The haemoglobin molecule contains no nucleus to maximize
space, however containing no nucleus does have its draw backs, in so much
as it shortens the life span of the molecule, typical human haemoglobin lasts
for about 120 days there are only scientific indicators as to how long fish
haemoglobin will last before being deselected and replaced by the spleen and
kidney, this will to some extent depend upon toxins found in the
water.
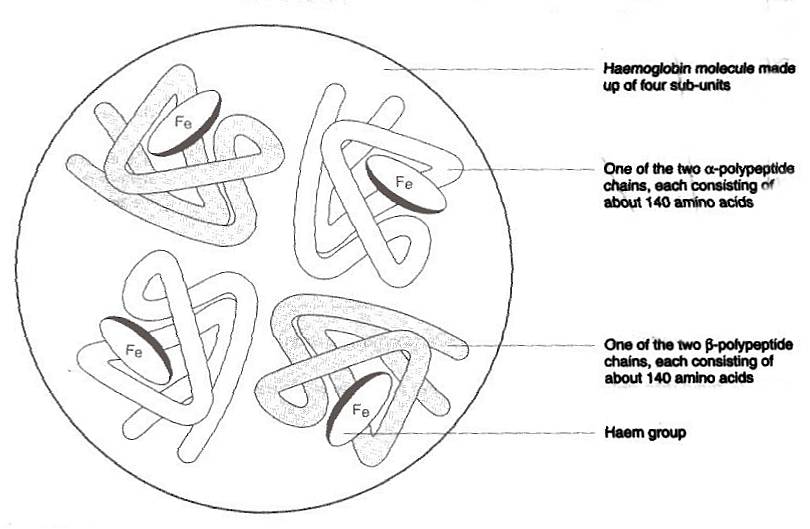
This phenomena, explains why the blood is capable
of holding and thus transporting 20 times
more oxygen than water can hold in solution. If the blood could only saturate
the plasma to the same levels that was held in simple solution in the pond
water there would be no need for the complexities of haemoglobin. Haemoglobin
is a simple but miraculous design.
So the blood is fully loaded and starts off on
its journey around the cardiovascular system to sites where it is needed,
i.e., tissue sites around the body where the oxygen is all but depleted. Once it reaches such a site, there needs to
be some kind of trigger to the haemoglobin to release the cargo (oxygen).
This is achieved in the following process.
The metabolic processes use up the available oxygen in the tissue. One of the results of this process is CO2.
Carbon dioxide dissolves into the body fluids as carbonic acid, thus there
is a lowering of the plasma pH in this area, where fresh Oxygen is needed.
Usually the typical pH of the blood is around 7.8 ph and can be governed to an extent on how low you run your
pond Ph, but at a tissue, site of much needed
oxygen, the pH can drop by 0.5. It
is this downward shift in pH that is the cue for the haemoglobin to drop off
its cargo of oxygen and upload the carbon dioxide and hold on to it. At the same time, carbonic acid diffuses into
the plasma and keeps the pH low on the blood’s journey back to the gills.
Because the oxygen in the tissue is very low, once the haemoglobin releases
the oxygen, this again causes oxygen to diffuse into the tissue from the higher
concentration in the blood. Also because
the blood arriving at the tissue has a low CO2 content, the CO2
diffuses into the plasma from the higher concentrations in the tissue.
Of the respired CO2, 5 % is carried
back to the gill dissolved into the blood plasma, a further 10% of the CO2
is held by the haemoglobin, attaching to the amino groups contained within
the haemoglobin molecule.
A massive 85% of the CO2 is carried back as carbonic acid dissolved
into the blood plasma
Once back at the gill, the carbon dioxide/carbonic
acid diffuses across the gill into the pond water thus raising the pH of the
blood. This clues the haemoglobin
to increase its uptake of oxygen and whole process begins again.
If however upon arrival at the gill, the blood
is met with a low pond pH, there will be a micro environment around the gill
lamellae that is even lower in pH due to the CO2 (converting to
carbonic acid) being released at the gill, the net effect of which is that
the haemoglobin will lose affinity for oxygen and tend to hang on to the CO2. This condition is termed “acidosis.” If the condition becomes chronic, compensation
by the fish usually takes the form of an increase in haemoglobin.
This
is an over simplified view but in essence this sums the process up.